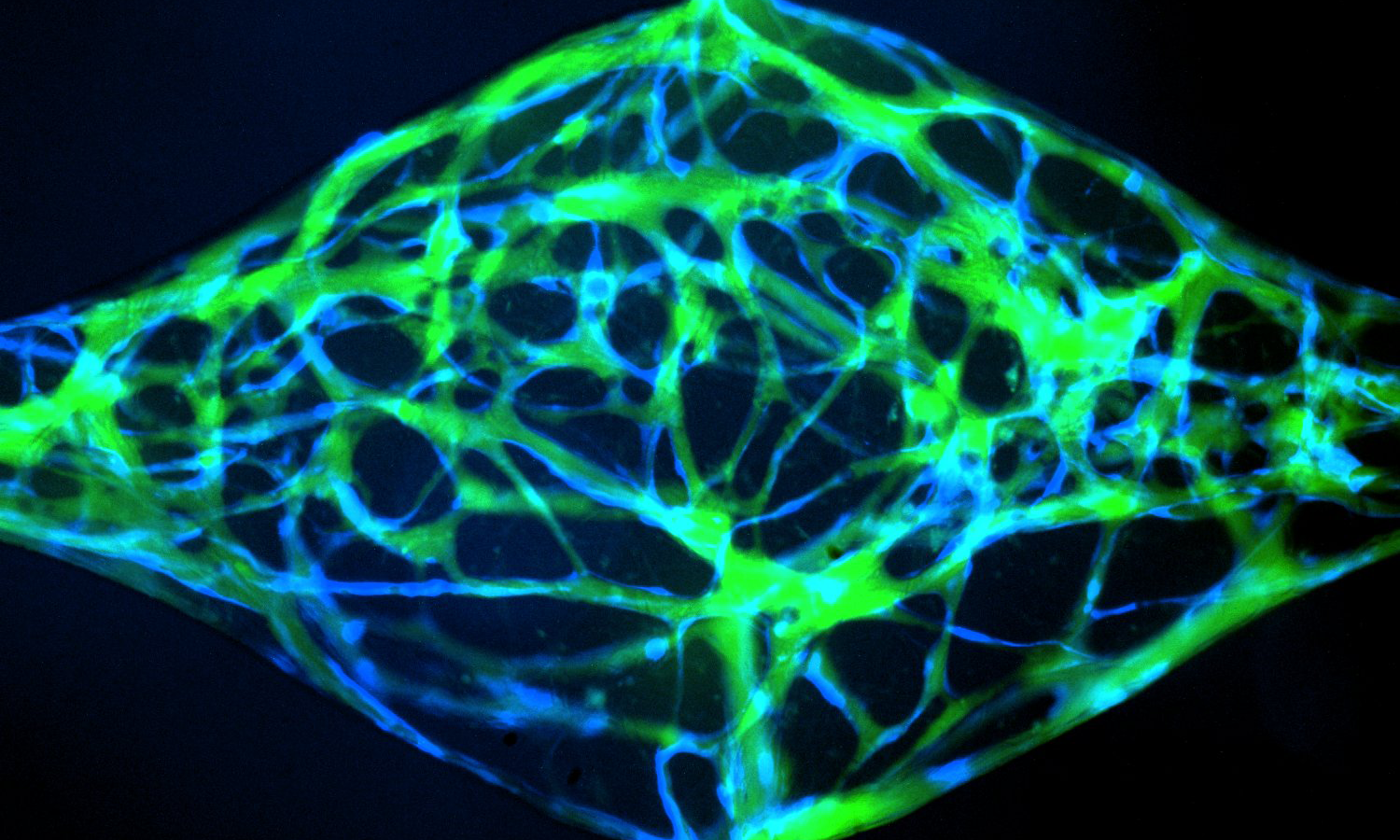
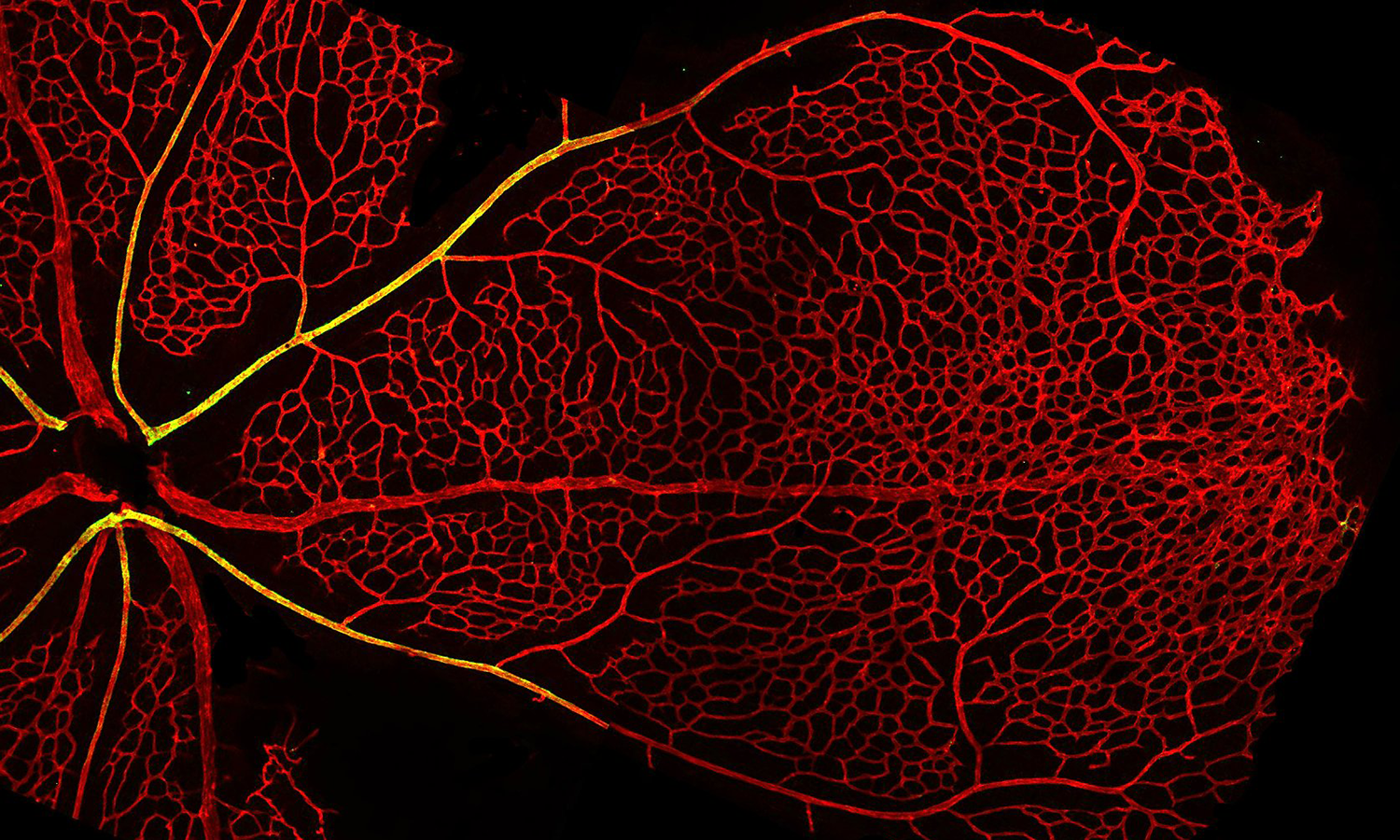
Dr. Fang leads a workshop on in vitro angiogenic assays
The Fang Lab
Our group is interested in better understanding how blood vessels grow, remodel, and reorganize during healthy tissue development to form mature structures such as arteries, capillaries and veins, and how this process might go awry in disease leading to disorganized and malformed blood vessels that can significantly compromise patient health. To address this question, we use a combination of in vivo animal models and novel microphysiological organ-on-a-chip microfluidic platforms to study how cells of the vasculature communicate with one another in growing and remodeling blood vessels.
One area of active research is to explore how cell-cell miscommunication leads to vascular malformations in the rare disease, Hereditary Hemorrhagic Telangiectasia (HHT). To study this question, we have developed the first HHT-on-a-chip microphysiological model, in which we are able to engineer artificial blood vessels to grow and remodel in a microfluidic platform. We use this model in conjunction with transgenic animal models to study vessel malformations over the course of their development.
Another area of interest is to study the regulatory signals that control sprouting angiogenesis during development and in diseases such as cancer. We are specifically interested in understanding the cell signaling pathways that control how endothelial cells enter and exit an angiogenic state, and how this might become dysregulated under pathological settings leading to uncontrolled vessel growth.